At Southcoast Health Heart & Vascular
Our cardiologists, cardiac surgeons, vascular surgeons and specialized NP/PAs provide professional cardiovascular care services with leading-edge techniques and advanced treatment plans that are customized to your needs.
Treating over 2,000 patients a year at one of the most active catheterization labs of any community hospital in MA and RI, Charlton Memorial Hospital is proud to be the region’s leader in world-class cardiovascular care, offering everything your heart needs – close to home.
The Cardiac Critical Care team works 24/7 responding to cardiac emergencies and have training in specialty areas, including critical care, cardiology, cardiac catheterization, cardiac anesthesia, and more. Southcoast Health provides advanced monitoring and support equipment, enabling the team to recognize problems promptly and respond effectively. If you need critical surgery or suffer a cardiovascular emergency, get on the road to recovery at the Heart and Vascular.

Our Services
General Cardiology
- General cardiology consultation (inpatient, outpatient)
- Heart failure
Arrhythmia Services
- Pacemaker and defibrillator implantation
- Atrial fibrillation wellness program
- Watchman device procedure
- Cardiac ablation
- Arrhythmia evaluation
- Ablation
- Medication management
Invasive/Interventional Cardiology
- Cardiac catheterization/coronary angiography
- Coronary angioplasty, stenting and atherectomy
- Coronary intravascular ultrasound and physiologic testing (Pressure wire)
- Percutaneous Left Ventricular Assist Devices (Impella)
Structural Heart
- Structural heart/valve clinic
- Watchman device procedure
- Transcatheter Aortic Valve Replacement (TAVR)
- Hemodynamic support devices (Impella) (cross linked from interventional)
- Adult congenital heart catheter based therapies (PFO, ASD closures)
Cardiac Surgery
- Open heart surgery
- Conventional and off-pump coronary bypass grafting
- Aortic, mitral and tricuspid valve repair and replacement
- Aortic aneurysm surgery
- Valve-sparing aortic root replacement
- Adult congenital cardiac defect repair
- Cardiac tumors
- Atrial fibrillation ablation procedures
- Transcatheter Aortic Valve Replacement
- Hemodynamic support devices (Impella)
- Robotic surgery, daVinci
Cardiac Rehabilitation
- Cardiac rehab
- Medical fitness
Non-Invasive Cardiac Testing
- Echocardiography
- Transesophageal echocardiography (TEE)
- Cardiac coronary CT angiography
- Cardiac MRI
- Cardiac stress testing – exercise stress test, stress echocardiography and nuclear stress testing
- Holter monitors
- Long-term event monitors
- Implantable loop recorders
- Peripheral vascular resistance testing
- Carotid Ultrasound
- Electrocardiogram (EKG)
Leading the Way in Patient Care and Life-changing Procedures
Southcoast Health Heart and Vascular was the first in the nation to perform a life-changing cardioneuroablation procedure.
Dr. Nitesh Sood, Cardiac Electrophysiologist and Director of the Atrial Fibrillation Wellness Program at Southcoast Health, performed the procedure on 32-year-old patient Jennifer Link of Middletown, R.I. Link was diagnosed with third degree – or complete – heart block this May after suffering from a lifetime of heart pauses – sometimes stopping her heart for as long as eight to 10 seconds.
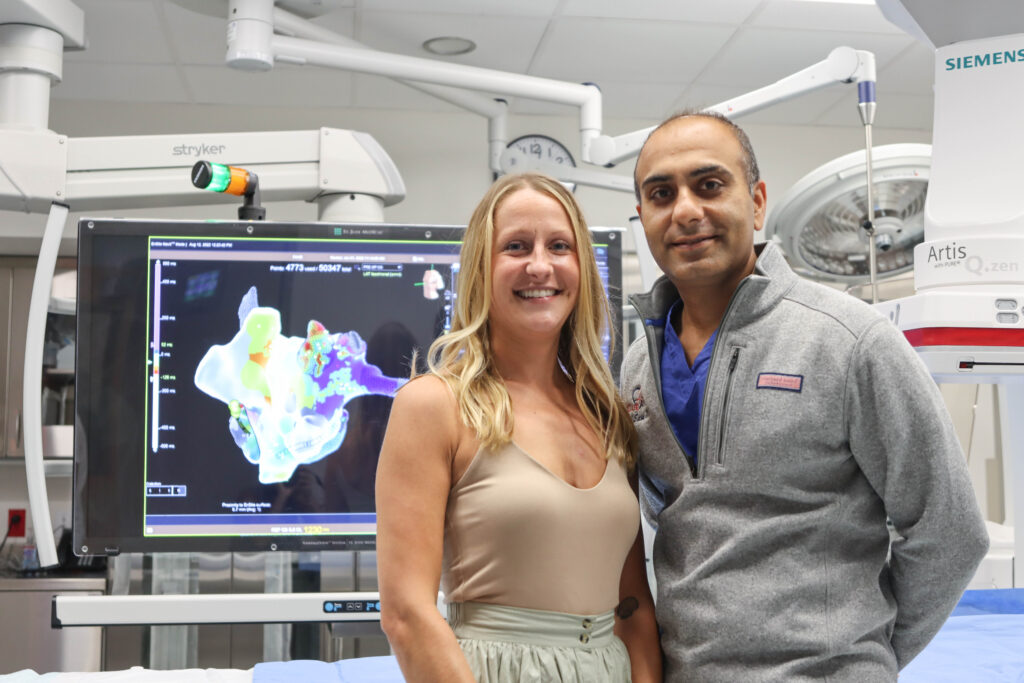
Real Patients. Real Results.




Find Your Cardiologist in MA & RI
At Southcoast Health, we understand that with a strong heart you can get more out of life. That is why we help your heart be as healthy as it can be. Our team includes cardiologists, cardiac surgeons, vascular surgeons and specialized NP/PAs who have years of experience in the treatment of cardiovascular disease.
Caring for the heart requires an insightful mind, and our board certified cardiologists, interventional cardiologists, electrophysiologists, structural heart specialists, cardiac surgeons, vascular surgeons are the best and the brightest. With exceptional training, our doctors deliver the compassionate, personal care that our heart and vascular patients deserve.
Find a cardiologist near you today for award-winning, individualized heart care.
Southcoast Health provides cardiac care in Fall River, Dartmouth, New Bedford and Wareham, MA and Rhode Island. We offer general cardiology consultation in our practices throughout the region.


